A WHOLE LABORATORY IN ONE DEVICE patented and CE certified
Discover our versatile real-time PCR system – the complete solution for your molecular biology analyses
These products are currently for exhibition and demonstration purposes only and cannot be made available until they have been made compliant with Regulation (EU) 2017/746 (IVDR)
One device for a wide range of applications:
- Comprehensive detection: Reliably detects all types of viruses, bacteria, and fungi using highly sensitive real-time PCR.
- Food control: Checks samples for contamination, such as insects in food.
- Flexibility thanks to test cartridges: We offer a wide range of specific test cartridges for your individual requirements.
Integrated sample preparation & measurement
Classic sample preparation:
The system includes everything necessary for standard sample preparation, including centrifugation capability and the required reagents.
Specialized cartridges:
Easy handling thanks to our specially developed test cartridges.
Precise results:
The system includes everything necessary for standard sample preparation, including centrifugation capability and the required reagents.
Specialized cartridges:
Easy handling thanks to our specially developed test cartridges.
Precise results:
Detection is performed via real-time PCR using fluorescence measurement, which delivers fast and accurate results.
Ideal for a wide range of applications, our PCR tester is perfect for:
- Research institutions
- Schools and universities
- Diagnostic laboratories
- Quality control and other fields of application
- Private use for testing
time-saving
Save
Save
Cost-effective
Cost-effective
Friendly support
Friendly support
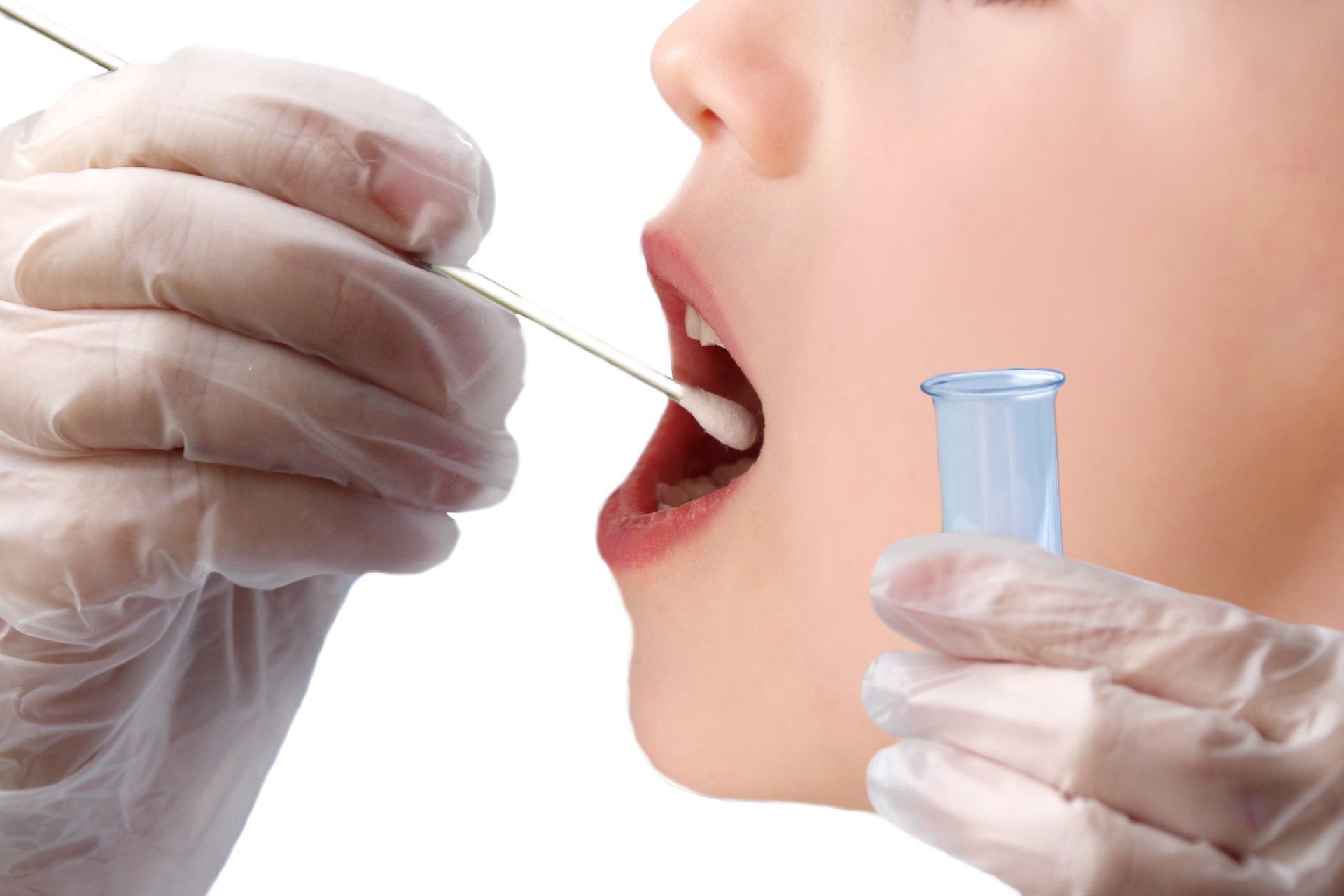
Take the test smear
Step 1: The sample is taken from a patient's nose and throat using a swab. Both swabs can also be taken separately. More detailed instructions on how to collect the sample can be found here.
Please note: One test sample is required per test person.
Bend the test stick and seal it
Step 2: Insert the test sticks with the swab sample into the test cannulas (elongated tube). The test sticks are then bent at the predetermined breaking point.
The test cannula should be closed with the help of the sealing cap.
Insert the sample into the test device
Step 3: Insert the test sample into the test channel of the
BionLyx test device.
BionLyx now prepares this test sample for about 2 minutes.
After this process, the test device automatically starts the analysis of the test sample of the person to be tested.
Attractive pricing model
Basic equipment:
Available for only €3,950
Test cartridges:
Available individually for €25 each
Basic equipment:
Available for only €3,950
Test cartridges:
Available individually for €25 each

Please contact us for further information or a customized quote!
FAQs
Do you have a question? We will be happy to help you.